
Our Sun Radiates Energy
The algae we see as green pond scum converts sunlight energy for its entire requirements at up to 95 percent efficiency, which is four times greater than solar photo-voltaic panels can manage.

Duckweed also known as water lentils or water lenses come from four species (Spirodela, Landoltia, Lemna, Wolffiella and Wolffia) are small floating aquatic plants. They are monocotyledons meaning a flowering plant that bears a single cotyledon (embryo seed leaf)1. The small round thin leafed plants seen on slow moving waters is very fast growing which tends to obscure its microbial and phytoplankton reduction, and high nutrient and metal accumulation characteristics.
When the water is frozen it lies on the bottom consuming its own starch. In mid spring it surfaces and there reproduces copies of itself. It can cover acres of water in a few months all powered by sunlight.
Joseph Priestly is known for his descriptions of the isolation and identification of oxygen and other gases such as ammonia, sulphur dioxide, nitrous oxide and nitrogen dioxide2. But in 1771 he performed a demonstration in a jar bell, placing in it a mint plant with a burning candle. The candle flame used up the oxygen and went out. After 27 days, Priestley was able to re-light the candle. He was not aware that sun light was the unknown ingredient. It was later that In 1779 Jan Ingenhousz found out that in the presence of light, plants give off bubbles from their green areas, while in the shade these bubbles stop. He determined this gas to be oxygen.
Our sun broadcasts vast amounts of energy in an array of wavelengths. Green plants, some algae and bacteria can use a small portion of that light to energize the process of separating the hydrogen from water (thus releasing oxygen). The hydrogen is then in a form to combine with carbon dioxide to produce a simple energy rich sugar. Animals (including us) use oxygen and the sugars to utilize the energy and emit carbon dioxide and water. These two processes occur every day, all day.
Our sun’s fusion of hydrogen delivers enough light energy to power all our needs without burning any fuel. Coal, petroleum oil, and natural gas are all created by compressing the remains of 600 million years of growth of plants and animals. The plants do the real work of converting solar radiation and storing it in greenery, grass, foliage and timber. To access that energy we burn the plant products; internally inside our cells or externally in fire. By burning the fossilized carbon fuels we release in one day what took one hundred thousand (100,000) years to form3.
The Mystery of Photosynthesis
IN 1912 Italian chemistry professor Giacomo Ciamican wrote in Science Magazine, he wrote of smoke stacks making way for ‘forests of clean glass tubes’ which would copy the ‘guarded secrets of plants’ using their natural processes to fuel our world.
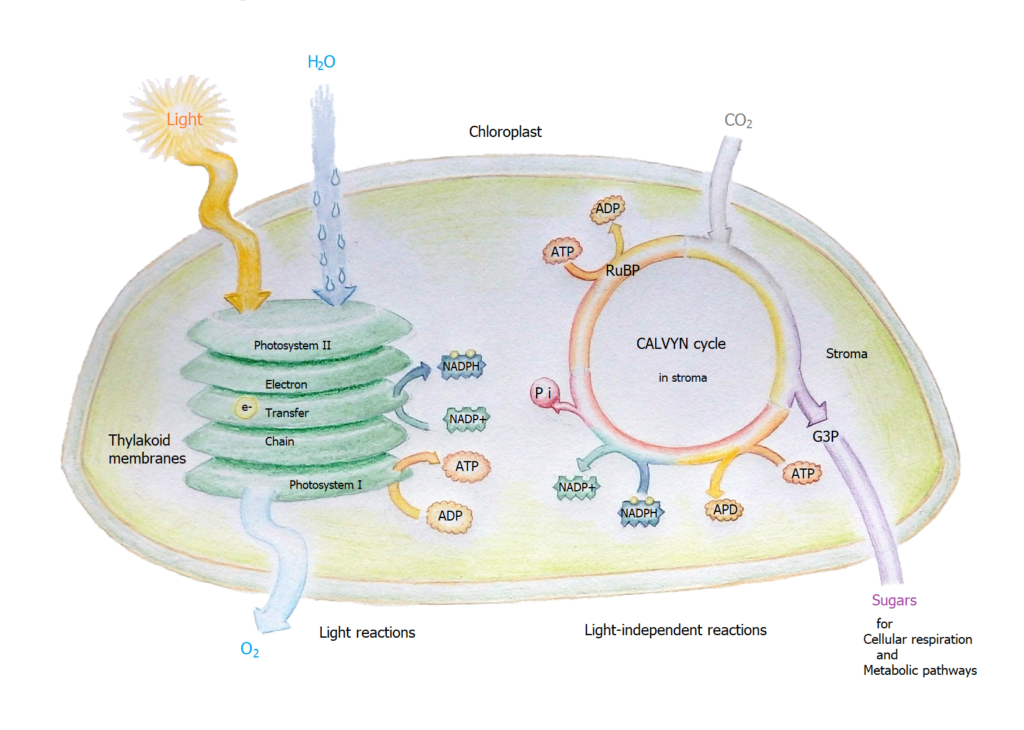
Man has not fully understood the process known as photo synthesis it remains tucked away in the molecular arrangement of atoms in cells referred to as chloroplasts It is all to do with being green. Pigments in chlorophylls and carotenoids are light sensitive. Molecules in the structure of the membrane of chloroplasts act like antennae and collect photons of energy.
Light to energy > How nature does it
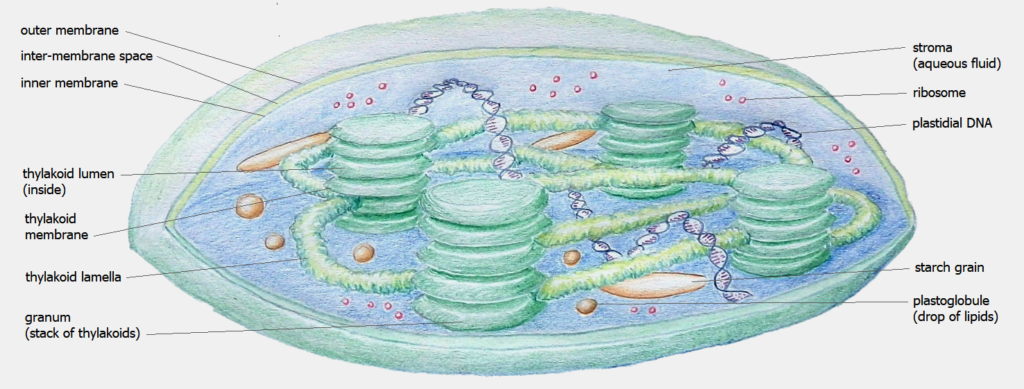
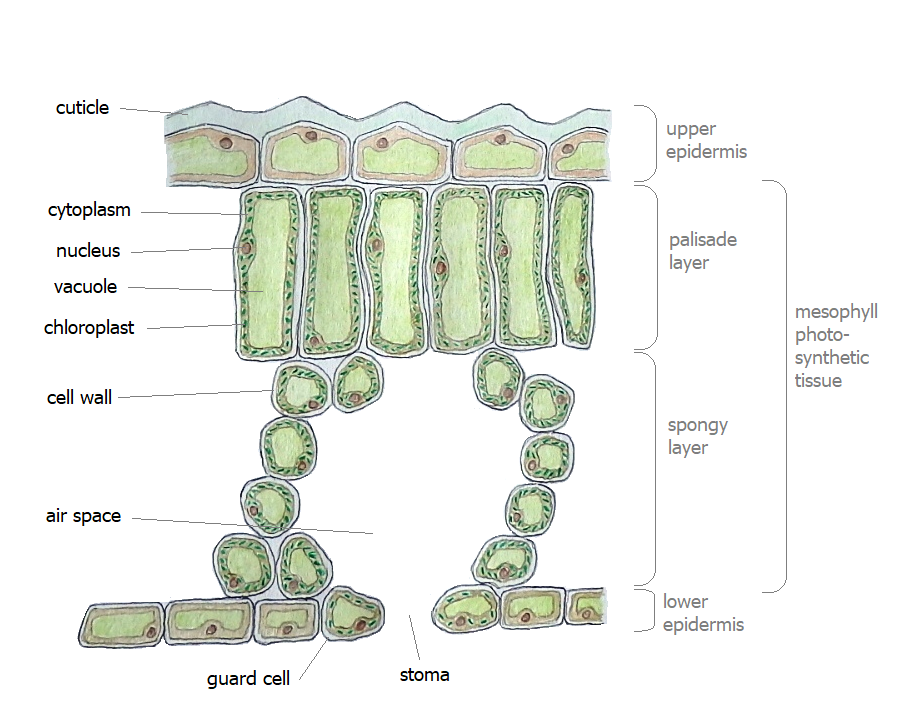
Inside a plant’s chloroplast inside its skin refered to as membrane are organs called a thylakoid. These thylakoids line up in a stack called a granum. Inside a thylakoid are thousands of chlorophyll molecules standing like a forest of tennis rackets awaiting a serve of photons. The various types of chlorophyll (a,b,c,d,e) absorb different wavelengths of light energy.
The broad end of the chlorophyll molecule is exposed to the photons and gets its electrons energized. This occurs in two photosynthesis systems simultaneously, we start with photosynthesis 2 (PS II).
PS II. A photon with the 680 nanometer wavelength (nm.λ ) contacts the chlorophyll antenna, an electron orbiting the chlorophyll molecule is energized into a higher orbit. When the electron is whisked away it is replaced by an electron from a ‘Z’ donator molecule. The energized electron jumps from acceptor molecule to acceptor molecule. Then it is promoted to the PS I where it meets at central chlorophyll.
PS I. The charged electron goes to a chlorophyll that has received a photon with the 700 nm.λ. The electron transfer keeps hopping to acceptor molecule to acceptor molecule eventually a charged electron is found outside the thylakoid membrane.
This is the difference between a sack of chemicals and a living leaf. The concentration of molecules and charge is different to the concentration and charge outside the membrane and can be referred to as a potential difference. The membrane provides the tension between unequal concentrations and charge.
The membrane potential accomplishes a lot in plants feeding and refueling the entire plant. Splitting water, giving off O2+ and leaving H+ inside the membrane to form NADPH,
H+ + e– + NADP+ –> NADPH So then outside the membrane,
Electron after electron are passed onto nicotinamide adenine dinucleotide phosphate (NADP+) transforming it into NADPH which has the ability to donate electrons to other molecular compounds.
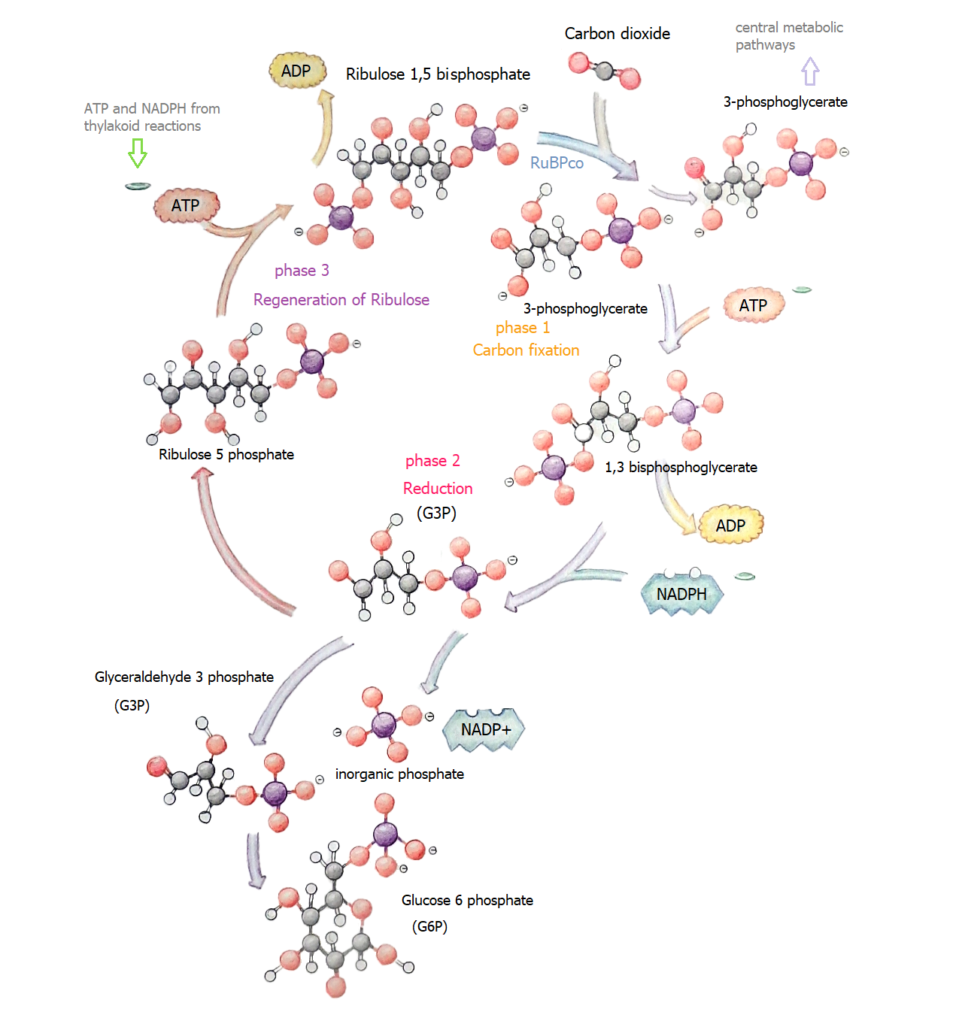
So NADPH donates an electron to carbon dioxide (CO2). However it needs energy to entice the H+ ions to form CH2O, sugar. [Now the potential difference either side of the membrane goes to work.]
2NADPH + CO2 –> CH2O (sugar) + 2NADH with the enzyme channeling the coupling factor
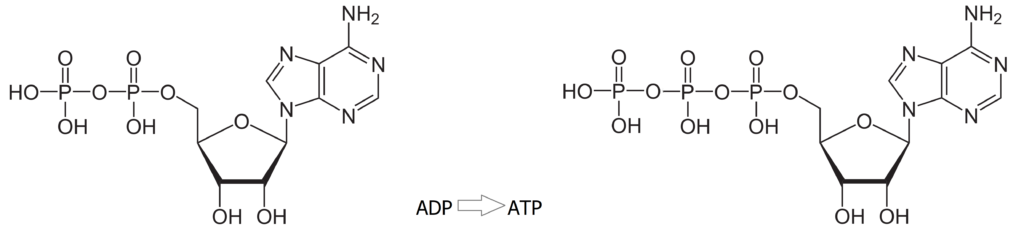
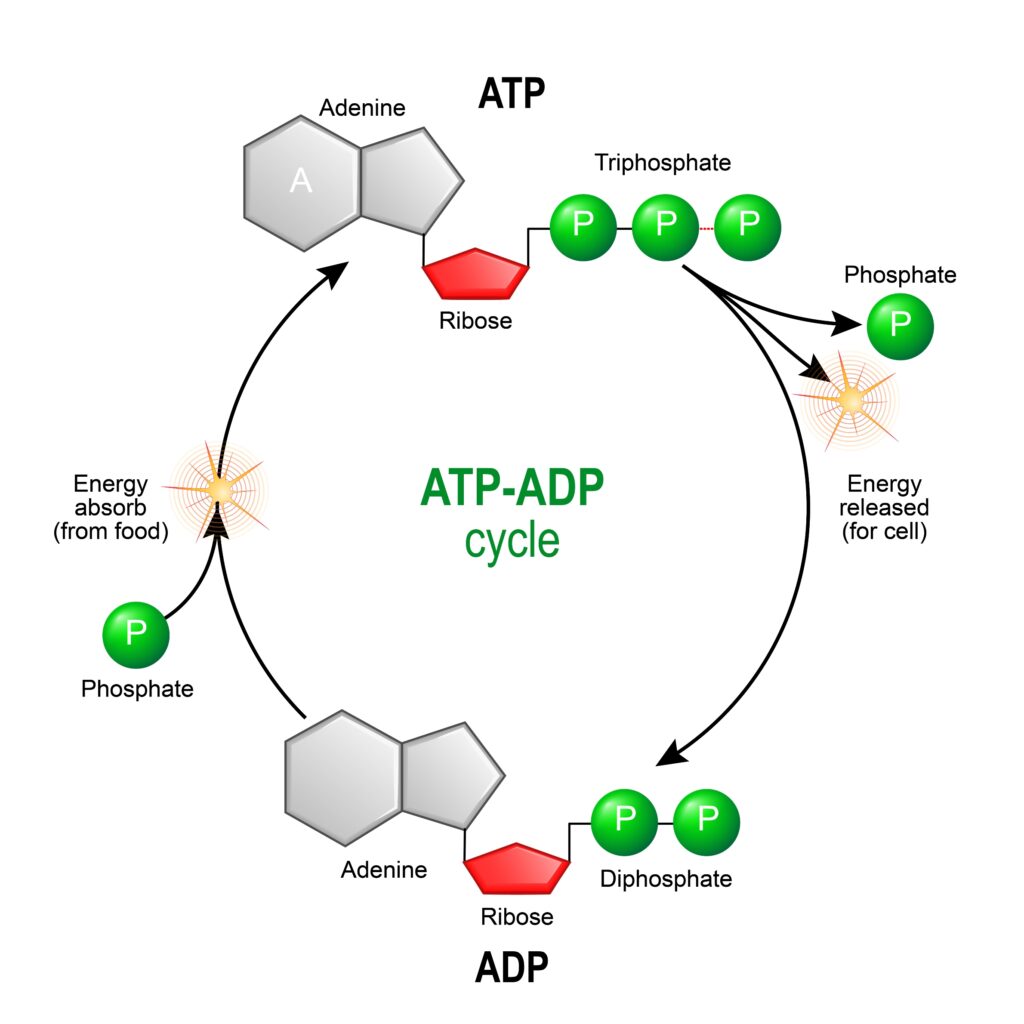
The hydrogen ion H+ gets through the thylakoid skin with an enzyme ‘channel’ referred to at a coupling factor. The positive charges escape through this coupling by turning Adenosine diphosphate, (ADP) to adenosine triphosphate (ATP) by adding the third phosphate to the other two with a high energy bond, which is the storage site of the energy gained from the sun’s photons.
Biochemist Thomas A. Moore has suggested applications of such an organic battery.
- Hooking a chain of dipolar molecules to create a current.
- Splitting water into hydrogen [a clean fuel] and oxygen.
- Use it as a power pack to power solar based manufacturing.
- Use it as a near light speed switch for computing.
Sustaining us today
Both animals and plants respire 4, I point this out to emphasis the cyclic nature of the whole ecological system.5 This diagram simplifies the process of cellular respiration.
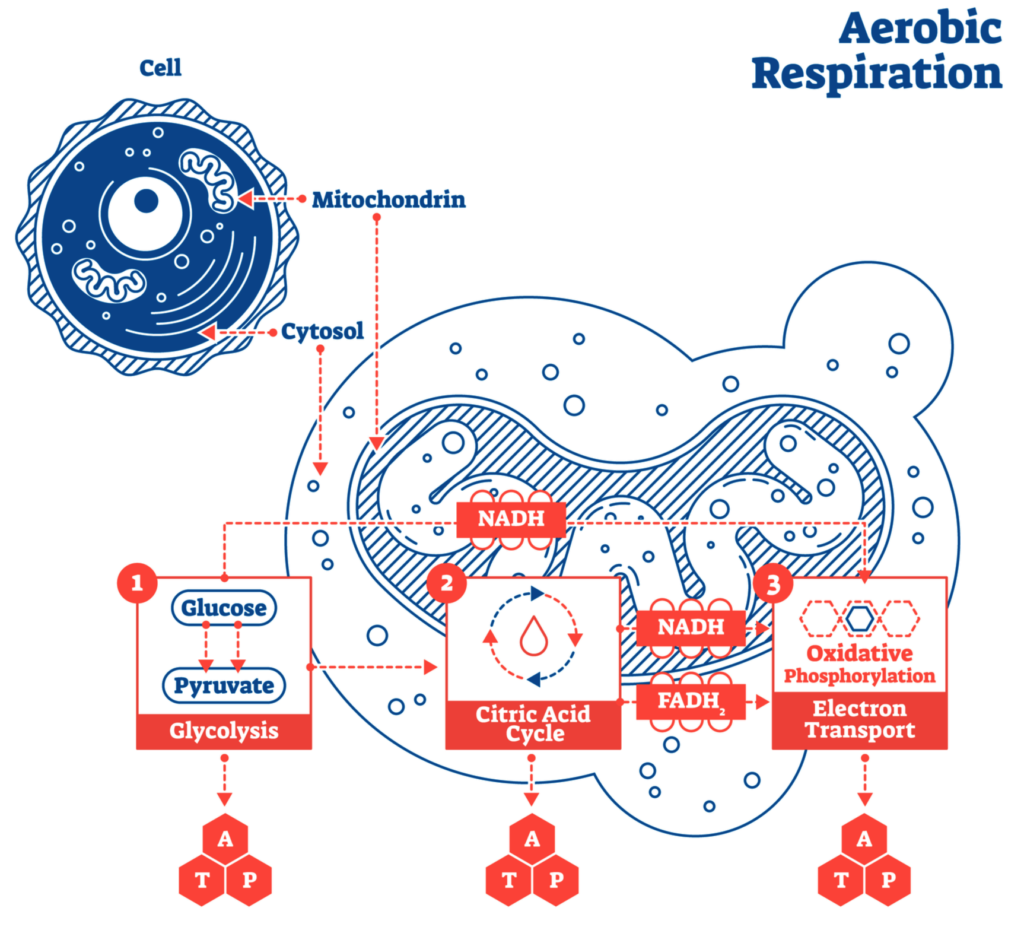
Photosynthesis produces 300 billion tonnes of sugar a year6,7. Within the thylakoid stacks called a granum is the reaction center which is composed of a number of molecular groups called ‘cofactors’ within a tangle of protein referred to as the ‘protein pocket’. The cofactor localities outline a wishbone shape with a chlorophyll pair at the center with cofactor limbs facing each other symmetrically. An electron uses the cofactor limbs to transfer through the membrane incredibly fast.
Purple bacterium Rhodopseudomonas viridis a sun harvesting microbe is structurally far simpler than green plants believed to be kin to the first photo synthesizers 3 billion years ago. It has fewer unknown black boxes in its photo synthesis system.8 The purple bacterium can switch its reaction center from photo synthesis to respiration, oxidizing its food as animals do.
Converting the sun’s energy is all about storing the energy like we do charging a battery [NiCd, lead-acid] with an electric current. The battery’s potential difference, its charge needs to be stable if it is to be able to do work for us like making a light glow or running a drill. The same applies to photosynthesis, that potential difference either side of the membrane needs to be stable long enough to do chemical work in forming sugar and starch which are chemical energy store houses.
Neal Woodbury a chemist works with x-ray crystallography studies the light reaction center of R. viridis. He works on modeling the reaction mechanism in the photo synthesis of light photons energizing the relocation of electrons. If you are not going to replicate the energy reaction center in thylakoids what molecules are you to use. Carotenoids are the pigment in the thylakoid’s antennae; porphyrins are similar to chlorophyll and also found in antennae. Some success was recorded when porphyrin was paired with an acceptor molecule quinone. The excited electron from porphyrin did move to orbit the quinine, but it extremely quickly returned to the porphyrin. Putting some distance between the charge differences seemed a strategy to employ.
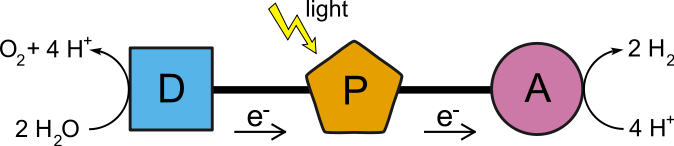
So a third molecule, a catalyst was added to the donor and acceptor molecules to have a triad like shown below. The photosensitizer-(P) accepts a photon charge to energize an electron which transfers to the acceptor a proton reducing catalyst-(A), and an electron is taken from the donor-(D) which gets it by splitting water into oxygen molecule and hydrogen ions.
The state of the triad with one catalyst oxidized on one end and the second one reduced on the other end of the triad is referred to as a charge separation, and is a driving force for further electron transfer, and consequently catalysis, to occur. The different components may be assembled in diverse ways, such as complexes with weak inter-molecular forces, compartmentalized cells, or linearly, covenant bonded molecules.9
The pental is a five part donor – donor – donor – acceptor – acceptor molecular line up. The chemical signature is C – PZn – P – Q – Qꙮ where C = carotene, PZn = Porphyrin with zinc, P = porphyrin, Q = naphthoquinone, Qꙮ = benzoquinone. It works when
- PZn receives photon energy.
- PZn loses its excitation to P.
- P transfers an electron to Q.
- PZn transfers an electron to P
- C transfers an electron to PZn , at the same time, Q transfers an electron to Qꙮ.
If the charge separation is spaced far enough, the natural tendency to return to its original location will be overcome.
For energy to be gained from the sun we need excitation to power electron transfer and hold the positive – negative charge (potential difference) in a stable location. The human species needs a means of storing high energy potential. Hydrogen is the choice in portable fuel cell development.
Getting hydrogen from water does not look difficult. Nature does it by photon excitation of electrons passed off to NADP+ then transforming into the electron donor NADPH. Provided we have H+ ions and a constant supply of electrons we have a clean fuel to collect.
Water does offer up its hydrogen in the presence of a hydrogenase (biocatalyst). However after a few hours the hydrogenase is exhausted by the availability of oxygen and the reaction ceases.
Computers store and transmit electronic bits (I or O) one signal at a time. In the generations to come there will be 3D networks of switches, the signal will be encoded in light wave lengths. They will need light sensitive switched, perhaps a devise like a pentad which changes its charge distribution (the electron – vacancy positions) in response to light frequency.
Membrane polarization – is common in all biological functions. Photozymes – James Guillet of the University of Toronto works on ways to get light to the reaction center. In plants it is done with pigment antennae. Sunlight is diffuse – like drizzle instead of hard rain. The energy units – photons are hard to collect. Plant leaves, algae, photosynthesizing bacteria all use antennae to attract photons to their reaction center. About 200 pigment ‘tennis racket’ shaped molecules turn their porphyrin ring to face the light rays.
When a photon hits anywhere in the array, it excites an electron in porphyrin to a higher orbit, the energy (not the electron) moves to an adjacent porphyrin ring poised to receive the energy. Like energy from sound waves vibrating nearby materials.
The second photon infusion – having so many antennae there is more chance of receiving that second energy hit in close succession, so that will power a change. A good reaction center is powerless without the photons arriving as needed, i.e. almost simultaneously.
Guillet has managed to get naphthalene chains to conduct photon energy with the aim of using the chains as energy conduits to a reservoir to hold enough energy to split molecules like breaking the bonds in H2O –> 2H + O2-. Guillet found anthracene to be an effective receptacle to put at the end of a naphthalene chain. It proved to be as good at converting photons to potential energy as natural photosynthesis antennae.
Natural life systems have some common tactics whether it exists in plants absorbing nutrients, flowers growing into fruit, or the working of a brain. They use chemicals in an aquatic solution acting as the best solvent. Industrialized man uses solvents although called organic only because they contain carbon, have proved hazardously toxic.
The problem with naphthalene molecules is they does not stay suspended in water very long. A solution is to attach some hydrophilic molecules to the chain of naphthalene forming a hydrophobic package. Soap exhibits contrary characteristics. Its molecule has one end that is attracted to water while the other end is not water accepting and groups with other water non-accepting ends of molecules forming a molecular spiky ball. These soap molecular spheres are called micelle. The water hating cores attract other water resistant molecules nearby like greasy stain molecules which gather with the water resistant centers of the micelles and are washed away from fabric fibers.
Guillets new polymers form pseudo-micelle with energy gathering at their core. These pseudo-micelle act like catalysts or enzymes – photozymes. PSSS-VN made of two Sodium styrenesulfonate and two vinylnaphthalene. Tested on pyrene and polychlorinated biphenyls (PCBs are resistant to breaking down in sunlight). The photozymes can attract the PCBs in low concentrations and break off the chlorine from the PCB rendering them a less toxic state for further biodegradation. Doing chemistry in water using sunlight is most effective at the upper layer and diminished with depth. Nature shows us a solution on most quiet waterways, the surface clouding algae. It was the infamous duckweed that inspired James Gillet to develop little dish shaped discs that float on water. The discs hold the biochemical ingredients to be excited by sunlight and react in water. The product is scooped up from the water when required.
Referenced footnotes
Ref 1: www.sciencedirect.com/topics/earth-and-planetary-sciences/duckweed
Ref 2: www.brlsi.org/joseph-priestley-man-who-discovered-oxygen
Ref 3: Hannah Ritchie and Max Roser (2020) – “Fossil Fuels”. Published online at OurWorldInData.org. Retrieved from: ‘https://ourworldindata.org/fossil-fuels’
Ref 4: West, John B. (1995). Respiratory physiology– the essentials. Baltimore: Williams & Wilkins. pp. 1–10. ISBN 0-683-08937-4.
Ref 5: Stryer, Lubert (1995). “Photosynthesis”. In: Biochemistry (Fourth ed.). New York: W.H. FreeMan and Company. pp. 653–680. ISBN 0-7167-2009-4
Ref 6: scienceillustrated.com.au/blog/features/2050-the-year-of-instant-oil
Ref 7: quizlet.com/173406978/chapter-7-photosynthesis-honors-biology
Ref 8: Deisenhofer, Johann and Michel,Hartmut. The photosynthetic reaction centre from the purple bacterium Rhodopseudomonas viridis. Biosci Rep. 1989 Aug;9(4):383-419.
Ref 9: Andreiadis, Eugen S.; Chavarot-Kerlidou, Murielle; Fontecave, Marc; Artero, Vincent (September–October 2011). “Artificial Photosynthesis: From Molecular Catalysts for Light-driven Water Splitting to Photoelectrochemical Cells”. Photochemistry and Photobiology. 87 (5): 946–964. doi:10.1111/j.1751-1097.2011.00966.x. PMID 21740444.
Further Reading
Related topics: Geo Thermal Energy – designfromnature, HYDROGEN – designfromnature

Awesome post! Keep up the great work! 🙂
Great content! Super high-quality! Keep it up! 🙂
Wow! This could be one particular of the most beneficial blogs We have ever arrive across on this subject. Basically Magnificent. I am also a specialist in this topic therefore I can understand your effort.
Awesome article. Will read on…
I am continually looking online for posts that can assist me. Thanks!
Hello there I am so glad I found your blog page, … now and would just like to say thanks for a marvelous post and an all round exciting blog (I also love the theme/design), I don’t have time to browse it all at the moment but I have book-marked it and also included your RSS feeds, so when I have time I will be back to read much more, Please do keep up the great work.
Awesome post. I’m a regular visitor of your web site and appreciate you taking the time to maintain the excellent site. I will be a regular visitor for a really long time.
Hydrogen is found in a number of forms, the Sun sprays hydrogen dust out onto any planetary body without a protective atmosphere. Our moon is covered with hydrogen dust.
Hydrogen is the simplest atomic element and consequently the earliest to form from available sub-atomic parts, protons neutrons and electrons. Human civilization is addicted to burning fuel for energy, hydrogen is the best choice to burn, i.e. combine with oxygen giving off the reacted product water vapour. Their is plenty of it, so it appears to be the ideal fuel. Thank you for your query.